PHILADELPHIA — On the fresh American Middle Affiliation assembly, a cavernous room filled with medical doctors erupted into applause. Cleveland Hospital heart specialist A. Michael Lincoff had simply introduced the dramatic — and from time to time enigmatic — result of a brand new medical trial at the weight reduction drug semaglutide.
The drug, offered underneath the emblem identify Wegovy, lower sufferers’ chance of primary cardiovascular issues via 20 p.c, Lincoff reported November 11. It additionally lowered the danger of death — from any motive — over the kind of 40 months sufferers participated within the learn about on reasonable.
Lincoff’s paintings notches but every other position for semaglutide, which medical doctors recently use to regard diabetes and weight problems. Earlier paintings had already exposed a cardiovascular receive advantages in other folks with kind 2 diabetes. The brand new learn about, which focused obese or overweight sufferers with heart problems, is the primary to turn that semaglutide can lend a hand the hearts of other folks with out diabetes too.
That’s vital as it expands the quantity of people that may have the benefit of the drug, says Tiffany Powell-Wiley, a heart specialist and epidemiologist on the Nationwide Middle, Lung and Blood Institute in Bethesda, Md. In america by myself, greater than 6 million overweight or obese other folks have heart problems however no longer diabetes. Semaglutide can be a recreation changer for those other folks, says Powell-Wiley, who used to be no longer a part of the brand new trial. Nonetheless, she and others on the assembly identified the restrictions of the learn about — and the restrictions of the drug.
Up to we would possibly desire a fast repair for the emerging ranges of weight problems observed around the globe, Powell-Wiley says, “it’s vital to remember the fact that this isn’t the panacea.” We nonetheless don’t know the way neatly semaglutide works in a various crew of other folks, she says. And the drug doesn’t repair the societal, environmental and social elements that result in weight problems — and its attainable well being penalties — within the first position.
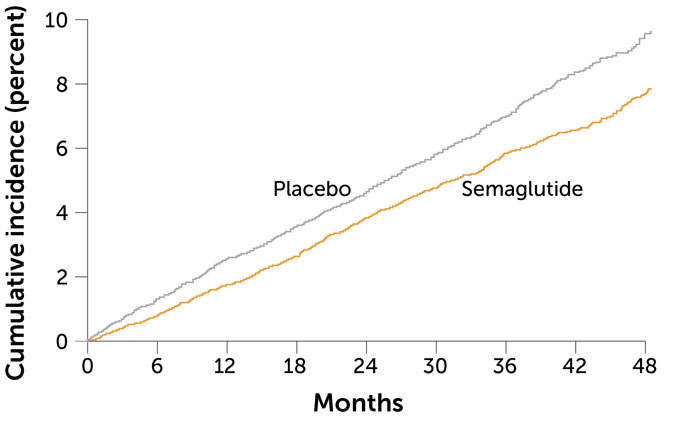
Semaglutide lowered the danger of middle assault, stroke and loss of life
Semaglutide is one in all a circle of relatives of substances rising in popularity for treating weight problems. On November 8, the U.S. Meals and Drug Management licensed a brand new one: tirzepatide, a relative of semaglutide that is going via the emblem identify Zepbound. [See also: Semaglutide FAQ ]
Semaglutide, offered underneath the emblem identify Ozempic, first got here available on the market for treating diabetes in 2017. Since then, the drug’s slew of makes use of has stored rising. In 2020, the FDA OK’d it for reducing cardiovascular chance in other folks with diabetes and middle illness. And in 2021, remedy for weight problems (with the upper dose Wegovy) joined the record.
Lincoff’s semaglutide trial, known as SELECT, integrated greater than 17,000 other folks, part of whom won weekly injections of semaglutide for kind of 3 years; the opposite part were given a placebo. Through the tip of the trial, 8 p.c of the folk within the placebo crew had had a nonfatal stroke or middle assault or died because of cardiovascular reasons. That quantity dropped to six.5 p.c within the semaglutide crew, Lincoff reported on the assembly and on-line November 11 within the New England Magazine of Drugs.
The variation between the 2 teams would possibly not sound like a lot, however “it’s a large outcome,” says Amit Khera, a heart specialist at UT Southwestern Clinical Heart in Dallas who used to be no longer a part of the trial. One can’t underestimate the significance of discovering a brand new remedy for sufferers with heart problems, he says.
The sickness is “the most important supply of mortality on the planet,” Lincoff mentioned at a information convention on November 10, and there’s no unmarried treatment that may do away with it. As an alternative, medical doctors attempt to chip away at it with quite a lot of therapies that provide incremental advantages.
For obese or overweight other folks with middle illness, Khera says, semaglutide is every other instrument in cardiologists’ toolkits.
Questions stay about how the drug works — and for whom
The brand new learn about effects flesh out previous information of the trial launched via funder Novo Nordisk in August (SN: 8/29/23). On the time, medical doctors sought after to grasp who the drug helped, the way it labored and what unwanted side effects it spark off. The most recent document gives solutions to a few questions however kicks others huge open.
Members on semaglutide had been relatively much more likely to drop out of the trial than the ones taking the placebo. That distinction gave the impression to be pushed via the drug’s gastrointestinal unwanted side effects, which come with nausea, diarrhea and vomiting. The collection of critical problems that happened, like most cancers or an infection, used to be relatively decrease within the semaglutide crew.
Despite the fact that semaglutide introduced transparent cardiovascular advantages for sufferers, one mysterious side of the information snagged researchers’ consideration. The drug’s protecting results changed into obvious early within the trial — neatly earlier than contributors had shed numerous kilos.
That’s a large deal as it suggests semaglutide is also boosting middle well being immediately, most likely along with the standard advantages that accompany weight reduction, says Caroline Apovian, an weight problems medication specialist at Harvard Clinical Faculty and Brigham and Girls’s Health facility in Boston. She used to be no longer concerned within the learn about however is on Novo Nordisk’s clinical advisory board. Lincoff mentioned his staff is “operating feverishly” on examining the SELECT knowledge to higher perceive semaglutide’s movements.
The ones knowledge come with demographic and clinical knowledge on hundreds of trial contributors. However the trial does have one “large limitation,” says Powell-Wiley: 84 p.c of the learn about’s contributors had been white and 72 p.c had been males.
Increasing the range of other folks represented in medical trials like those is a very powerful, she mentioned. It’s some extent she introduced up in a panel dialogue after Lincoff’s communicate, and one who — just like the trial’s effects — garnered common applause. In america, African-American, Hispanic and Indigenous individuals are maximum impacted via weight problems, Powell-Wiley says, and the brand new trial doesn’t let us know a lot about how semaglutide works in the ones teams.